The year is 420 B.C. Greece. The sun is setting in the far west. A Greek named Democritus is sitting and watching the beautiful Red sun, set in the sea. He has a mug in his hand. He is drinking water. He thinks to himself, what is this water made up of? He starts wondering and suddenly the mug slips all the water is spilled on the ground. Splash!!! What he sees on the ground startles him! He sees that as the water falls, it breaks into tiny droplets. As water touches the ground, it spills again and breaks into smaller parts. So he fills water again in the mug and spills it, deliberately this time. Again he wonders at how the water breaks into droplets. His hand being wet, he even drops the mug. The mug made of mud also breaks into pieces. Thud!!! He starts smiling to himself. He crushes the broken parts of the mug again. Smaller pieces!!! He wonders how small he can go. As small as the grains of sand around him? He continues breaking several things and finally makes a proclamation that matter is composed of tiny, indivisible particles in constant motion. These indivisible particles he calls “atomos” which means indestructible particle. As it happens with many great ideas, his idea dies with him.
It was not until 1803, a 36 year old chemist was trying to find out the answers to questions like “Why did (say) one gram of hydrogen always combine with 8 grams of oxygen, never more, never less?”, that Democritus’s idea was given more thought. He kept daily weather records. He was very interested in meteorology. He was John Dalton. In the course of his studies on meteorology, Dalton concluded that evaporated water exists in air as an independent gas. He wondered how water and air could occupy the same space at the same time, when obviously solid bodies can't. If the water and air were composed of discrete particles, Dalton reasoned, evaporation might be viewed as a mixing of water particles with air particles. These particles he called atoms from “atomos” again. Now atoms were the smallest particles known to man. Mark this statement again:
“Atoms were the smallest particles known to man”.
Let’s number this statement as 1. We will be using this statement to make an interesting notion.
Now let’s go a bit further with the history in hand.
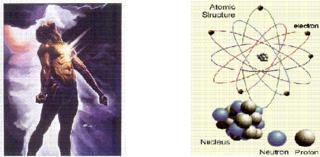
There have been dedicated researchers in all centuries. Here I am talking about a British physicist, who changed the very idea of construction of universe. Until him, all matter was made up of indivisible particles called Atoms. Around one hundred years ago amidst glowing glass tubes and the hum of electricity, this British physicist J.J. Thomson ventured into the interior of the atom!!! The particle supposed to be the building block of all matter and which was considered indivisible. At the Cavendish Laboratory at Cambridge University, Thomson was experimenting with currents of electricity inside empty glass tubes. He was investigating a long-standing puzzle known as "cathode rays." His experiments prompted him to make a bold proposal: these mysterious rays are streams of particles much smaller than atoms; they are in fact minuscule pieces of atoms. He called these particles "corpuscles," and suggested that they might make up all of the matter in atoms. It was startling to imagine a particle residing inside the atom-the indivisible, the most fundamental unit of matter.
After several more experiments (Lets spare the details of all the experiments) and suggestions from fellow scientists, he made a hypothesis:
“We have in the cathode rays matter in a new state, a state in which the subdivision of matter is carried very much further than in the ordinary gaseous state: a state in which all matter... is of one and the same kind; this matter being the substance from which all the chemical elements are built up."
The subdivided particles were called as corpuscles by Thomson. Irish physicist George Francis FitzGerald, in 1897 named these corpuscles as “electrons”.
So,
“Electrons were the smallest particles known to man”
Let’s again number this statement as 2. We will be using it for our notion. So let’s try and remember this also.
Okay let’s continue our journey through the past.
Year1896, month march in France it is was overcast weather. A local physicist Antonie Henri Becquerel was conducting experiments which needed sunlight. But since the weather he couldn’t get the sunlight he was looking for. He had among his apparatus, photographic plates and some uranium. Physicists are known to carry weird substances.
Okay let’s not deviate from the story. He kept both of these in a darkened drawer. Much to his Becquerel's surprise, the plates were exposed during storage by invisible emanations from the uranium. The emanations did not require the presence of an initiating energy source--the crystals emitted rays on their own! As John Lennon puts is so wisely”Life is what happens to you while you're busy making other plans”. By accident what he had found out was what is today popularly known as Radioactivity.
This serendipitous discovery of Radioactivity was further pursued with great interest by many scientists. These studies lead to some astonishing discoveries; I would rather call them findings rather than the former.
Ernest Rutherford was a New-Zealander with a Scottish origin. He was a student of Thomson and a great physicist. He conducted some war type experiments and bombarded the atoms with fast moving Cathode-Rays. What do we call this-A war against the building blocks? Whatever we call it, his experiments led to the discovery of what we call nucleus now and that this bulky thing in the atom constitutes all the weight and positive charge of the atom.
So referring to statement 2, it was not only electrons that made up the atom. There were some more particles!!! These are the particles that make up the nucleus, which were further named as protons and neutrons, positively charged and electrically neutral respectively. Electrically neutral? Sometimes these physicists are really confusing. They say electrons move and thus produce electricity and then make a statement like “Neutrons are electrically neutral” when they also say that nucleus where neutrons are found don’t contain any electrons. Interesting, but still true!!!
Okay now Man knew three fundamental particles negatively charged “Electrons”, positively charged “Protons” and electrically neutral “Neutrons”.
Now again let’s mark this statement as 3. This will again be used in our future notion.
Okay I know it has been too much of physics and chemistry in this story.
Time to take a break!!! Just remember the three statements and have some coffee before starting this next part. You might be wondering, “Where is this weirdo getting to with his boring physics and chemistry stories?” I promise it will be worth all this waiting and coffee. We are reaching that illusive notion I have been talking about all along.
It was in 1956, Fred Reines of the University of California at Irvine and the late George Cowan were working on the beta decay of the nucleus and they found the particles named neutrino. Further experiments proved the existence of smaller particles generally classified as Leptons, neutrino being one of them. In July 1998, it was proved by a joint venture of US and Japanese scientists that these neutrinos are one of the fundamental particles and have a mass lesser than an electron. I mean, where was man heading? A particle smaller than an electron! That’s really mind blowing. It is also said that millions of these pass through the body every second. Why don’t these leave a dent? Well it’s again a mystery which is out of the scope here.
So now we had these funny named neutrinos as the smallest particles known to man.
So the elementary particles or the fundamental particles were these Leptons which included neutrinos, electrons etc and the protons and neutrons which are collectively called Baryons.
Let’s not mark this. Because as Sherlock Holmes says, “Mind is like an attic”. So you must keep only things necessary in it.
Okay going forward, there is a lab named Fermi lab. This lab by around 1995 found out that, protons and neutrons are not the fundamental particles, In fact they are made up of smaller particles called Quarks. Just the thought of this is sickening me. Just take a look at the names of these small particles:
Down, Up, Strange, Charm, Bottom and Top.
Isn’t that interesting? Well now where are we in the history of the atom and its structure?
Okay let’s mark the next statement also, as statement 4
The Fundamental particles are Leptons and Quarks. Leptons comprise of the electrons and the almost mass less Neutrino. Quarks are the particles of which protons and neutrons are made of.
Ufff…I am tired of finding more microscopic particles. Let’s stop here in our research about the sub-atomic particles. We have enough data to come to our notion.
God… Who is this god or what is this god? I will take the definition used by many scientists; God is a power behind the nature’s creation. Okay so God built us this beautiful Universe. So God must be very very big!!!
Wait a second! Let’s analyze this properly, what did God actually make? Go to statement 1. Atoms were the smallest particles known to man in around 1803. So we knew matter is made up of atoms. Who made these atoms? GOD as there was no better answer for it.
Then in about 1897, we came to know (statement 2) that atoms are not the fundamental particles, but they are made up of electrons. Referring to statement 3, in the earlier part of the 20th century, Atoms was in fact made up of protons, neutrons and electrons.
Who made these particles? Again it was GOD, the almighty. Interesting word people use to describe GOD, the almighty the most powerful. He created the smallest particles of nature known then, protons, neutrons and electrons and everything else was made of these indivisible particles.
In the latter part of the 20th century, the 90’s, we came to know that protons and neutrons are further made of Quarks (statement 4).
So from statement 4, Quarks and Leptons are the most fundamental particles known to man. Who created these again, GOD!
A wild dog has a den and a sparrow has a nest. Both built them on there own. The size depends on the size of the maker. So the den is larger than the nest. Using this hypothesis (Sorry for being so mathematical), I want to make forward a notion that…
GOD IS SHRINKING!!!
In nineteenth century he was so big that he created the atom, and by the end of twentieth century he is so small that he is creating Quarks and Neutrinos. God is the creator, but his size is shrinking as our knowledge about the atomic structure is increasing. Man is a logical animal. This is my deduction of the study I made about the belief of what god is and the atomic structure.
So, God is shrinking and our knowledge is increasing. How good it is for the mankind, Time is the only thing that will tell.


1 comment:
hmm.. very interesting!! the data and the knowledge given in the first half( the 4 statements) was really Gr8. i felt as if I was reading a RC passgae in a CAT exam. very well written
Regarding the second half which involves God...
Acc to me, god is a myth! and as is the case of 'Myths' - they dimnish as the knowledge increases. so the concept called GOD will fade away as the knowledge increases - knowledge that there is a specific scientific reason behind everything!!! i dont know if i have got u ryt but that is what I think..
Post a Comment